Why transglutaminase is the catalyst of food production
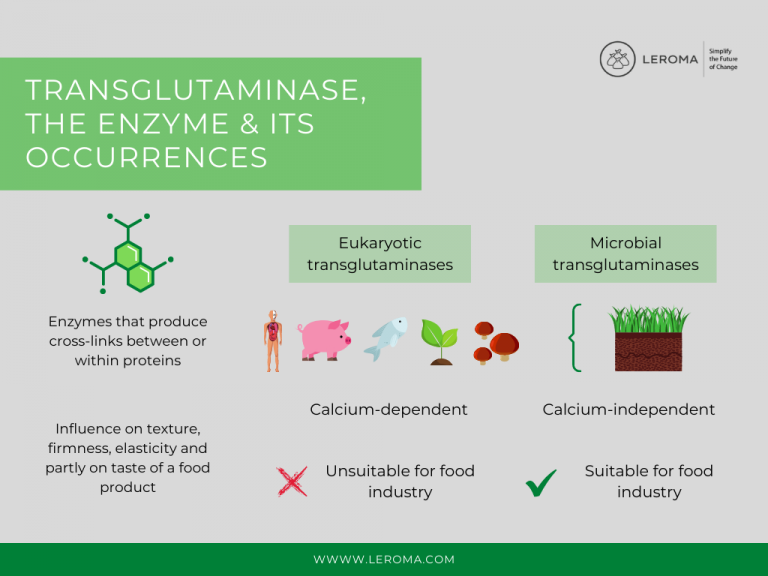
Transglutaminases (protein-glutamine-γ-glutamyltransferases) are enzymes that generate crosslinks between or within proteins. They polymerize, i.e they solidify, thereby strongly modifying the properties of the substrate in which they act.
Transglutaminases are of natural origin and are found in almost all
eukaryotes, organisms whose cells have a nucleus. These eukaryotes include humans, animals such as poultry, fish, pigs and cattle, as well as plants and fungi. Humans alone produce eight different transglutaminases. They act as biocatalysts and stimulate chemical reactions in our organism, such as blood clotting, in which fibrin is solidified, or tissue formation.
In addition to eukaryotic transglutaminases, there are also
microbial transglutaminases, which are mainly obtained from the bacterium "Streptomyces mobaraensis", which occurs predominantly in soil and has a distinctive, earthy odor.
An important difference between the two variants is their calcium dependence. The eukaryotic transglutaminases are calcium-dependent and are not suitable for use in the food industry because protein-rich foods often contain calcium, which can inhibit activation of the enzyme. Microbial transglutaminases, on the other hand, are calcium-independent and can therefore serve as an effective additive in the processing of protein-rich foods.
Transglutaminases (and their application) in the food industry
Transglutaminases have a major influence on the texture, firmness, elasticity and, to some extent, the taste of the end product. They can therefore change the physical properties of protein-rich foods and are mainly used in the production of meat, fish and dairy products. In addition, microbial transglutaminase is considered as
clean label and does not have to be listed on the label of the final product.
In the following text, we would like to introduce the application areas of transglutaminase. We replace the term "transglutaminase" with the common abbreviation
TG.
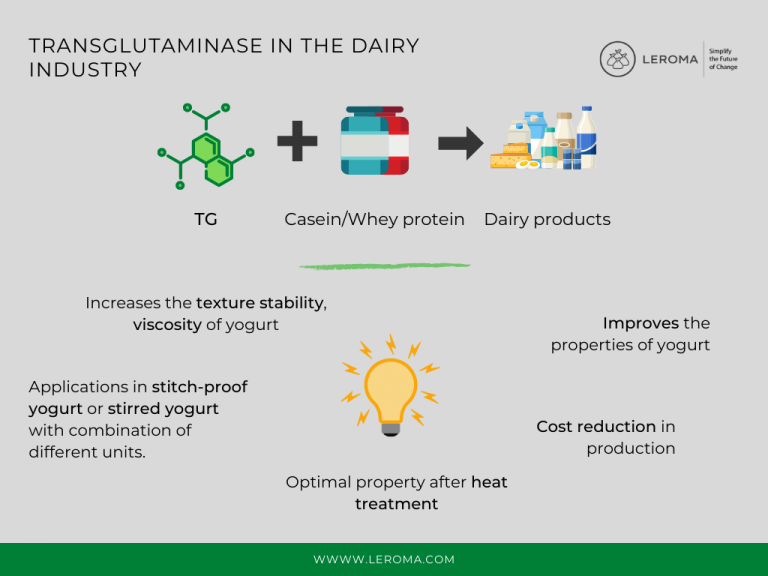
The application of TG in dairy products
In dairy products, TG reacts with caseins and whey proteins, with caseins being the more effective substrate due to their open tertiary structure. Sodium caseinate is particularly well suited. Caseins are often used as natural emulsifiers and their emulsifying properties are further enhanced by reaction with TG. Whey proteins have a quaternary structure, but can improve their properties as a substrate for TG by heat treatment or using a reducing agent. In general, among whey proteins, alpha-lactalbumin is more effective than beta-lactoglobulin.
So far, TG has been applied mainly to improve the quality of yogurt and cheese.
TG increases the texture stability of
yogurt during transport as well as its viscosity, giving it a smoother and drier surface. It also helps lower the cream content and provides a slightly whiter color, but most importantly, it does not change the natural taste of the products compared to other stabilizers.
In the following, we take a brief look at two application examples of TG in yogurt.
- In
set yogurt, TG can increase the gel strength. To do this, one percent of skimmed milk powder (SMP) is replaced by 150 ppm TG with an activity of one hundred units per gram. This results in a 22 percent increase in gel strength and a 7.4 percent reduction in syneresis.
- For
stirred yogurt, one percent SMP can be replaced by 305 ppm TG with an activity of one hundred units per gram. This increases viscosity by 61.4 percent and reduces syneresis by 16.1 percent.
In addition to improving the properties of yogurt, TG also helps reduce the cost of production, as the price of 150 ppm TG is significantly lower than that of one percent SMP.
Experience has shown that TG is best added after heat treatment in the dairy product manufacturing process, as whey proteins are denatured at that point, meaning the structure is more open and accessible to TG. Non-thermal technologies such as HHP (High Hydrostatic Pressure) can also be used in the processing of TG.
It is also possible to treat plant based foods with TG. For example, plant milk can be coagulated with TG to produce vegan cheese.
The application of TG in meat products
In the meat industry, TG is mainly used in the production of molded meat.
In this process, smaller pieces of meat are assembled into a larger product so that they can be used not only for sausage and minced meat, but also for cooked ham, cutlets and the like. To produce molded meat, the pieces are tumbled so that the muscle fibers loosen and protein emerges from the surface. Enzymes such as transglutaminase may be added during this process. The mass is pressed into the desired shapes and frozen or heated until the protein coagulates and the small chunks of meat are thus joined into one piece. TG supports the coagulation process in this step. Because of this function, TG is also referred to as "natural glue".
However, the meat industry does not limit TG to its function as a glue, but also uses the enzyme to produce a crunchier bite in sausages or better slice cohesion in sliced cooked ham.
The application of TG in bakery products
The activity and purity of the TG enzyme as well as the type of packaging may vary depending on the manufacturer. The differences affect the final product, so the choice of supplier should not be underestimated. In addition to dosage and enzyme activity, there are external factors that can affect the quality of the end product and should be taken into account during storage and processing:
The activity of TG is reduced by
oxygen, which is why the enzyme must be kept as airtight as possible during transport and storage. After unsealing the packaging, it is therefore advisable to use up the enzyme, which is usually sold in
powder form, as soon as possible. Dissolved oxygen, which is contained in milk, for example, should also be reduced as far as possible.
In addition, attention must be paid to the
temperature, which is ideally 50 °C, in order to achieve the maximum activity of TG. Below 2 °C and above 65 °C, TG is inactivated, sometimes irreversibly. TG can also be inactivated when the
pH value is below 4.5. Optimally, the substrate treated with TG has a pH between five and seven, below which the activity of the enzyme decreases. Finally, the function of TG can also be limited by various
inhibitors. These include, above all, the heavy metals lead, zinc, copper, nickel, cobalt and iron.
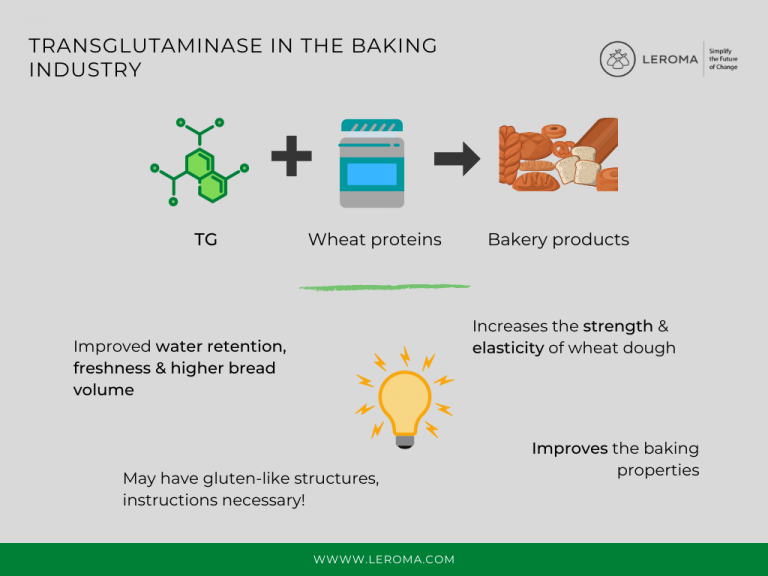
TG can be used to improve baking properties. The enzyme increases the strength and elasticity of wheat dough by reacting with wheat proteins. In addition, TG is said to optimize the protein structure of rye dough and contribute to improved water binding and freshness maintenance as well as increased bread volume.
Food manufacturers should note that the reaction of dietary proteins with TG can result in compounds that have gluten-like structures. Thus, the food products produced may be unsuitable for consumers who are allergic to gluten. This should be stated on the label of the final products.
Having looked at the areas of application of transglutaminases, we will now look at the properties and factors that influence the industrial use of TG:
Dosage is particularly important, depending mainly on the protein content of the product to be improved. In general, the higher the protein content, the more TG should be used. TG manufacturers recommend an amount of 150 ppm TG with an activity of 100 units per gram at a protein content of about 3 - 3.5%. (0.5 units of enzyme per gram of protein.) This leads us to the
enzyme activity: on the market you can find transglutaminases with different activities such as 100, 300 or 1000 units per gram. If an enzyme with high activity is used, the required amount is naturally lower than for an enzyme with low activity.
The use of transglutaminase has increased in recent years, especially in the dairy industry, and due to its positive effects, the enzyme will remain an important tool throughout the food industry in the future. Of course, you can also find transglutaminase on LEROMA`s platform.
Let‘s simplify the future of change!